A Review of Potential Adverse Effects of Long-term Opioid Therapy
- Protocol
- Open Access
- Published:
Relative frequency and take chances factors for long-term opioid therapy following surgery and trauma amongst adults: a systematic review protocol
Systematic Reviews volume 7, Article number:97 (2018) Cite this article
Abstruse
Background
When patients have been on opioid therapy for more than ninety days, more than than half of them continue using opioids years later. Knowing that long-term opioid consumption could atomic number 82 to harmful side effects including misuse, abuse, and habit, it is important to understand the risks of transitioning to prolonged opioid therapy to reduce its occurrence. Perioperative and trauma contexts are ideal models commonly used to report such transition. Long-term apply of opioids might be associated with transformation of acute pain to chronic, which might be an case of a risk cistron. The objectives of this knowledge synthesis are to examine the relative frequency and the adventure factors for transitioning to long-term opioid therapy amidst patients who have undergone a surgical procedure or experienced a trauma.
Methods
The proposed report methodology is based on Preferred ReportIng Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) statements on the conduct of systematic review and meta-assay, the MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies, and the Cochrane Handbook for Systematic Review of Interventions. A systematic literature search will include multiple databases: Cochrane Central, EMBASE, MEDLINE, PsycINFO, CINHAL, PubMed, and the greyness literature. We will identify studies related to opioid use beyond acute/subacute hurting control afterward surgery or trauma. Two of the reviewers will screen all retrieved manufactures for eligibility and data extraction then critically assess all identified studies. We will compile a narrative synthesis of all results and deport a meta-analysis when feasible. As available data permits, we will perform a subgroup analysis of vulnerable populations.
Give-and-take
This systematic review will contribute to the prevention and harm reduction strategies associated with prescription opioids past identifying take chances factors leading to the unwarranted long-term opioid therapy. The identification of mutual risk factors for long-term opioid therapy will help to orient further research on pain management likewise equally offer key therapeutic targets for the development of strategies to prevent prolonged opioid apply.
Systematic review registration
This protocol was registered in PROSPERO on March 2, 2018; registration number CRD42012018089907.
Introduction
Opioid prescriptions accept significantly increased over the last two to three decades, especially in the The states and across Canada [i, 2]. Opioids remain amongst the most widely used analgesic medications in the treatment of chronic non-cancer pain (CNCP). Post-surgical pain management is a swell example of nearly systematic use of opioids as fourscore% of surgical patients are prescribed opioids [three]. Indeed, untreated postoperative pain could have detrimental consequences such as development of CNCP, deterioration of quality of life and poorer medical outcomes [iv]. It is non rare that opioid therapy is initiated without a articulate handling plan, and prescriptions are renewed when patients complain of persistent pain thus leading unintentionally to prolonged opioid therapy [five]. This practice has numerous societal implications since the storage of unused medications [6, seven], improper discarding practices of excess narcotic analgesics [8] and/or used medications (e.thou., fentanyl patches), and deficient inter-professional person communication (e.thou., family doctors and specialists) [9] could pose a public wellness threat. This may be complicated by diverse non-coordinated trials of opioids past unlike providers, some of which may exceed recovery time, in addition to the insufficient patient follow-upward and elevated caseload of many clinicians.
In this context, long-term opioid therapy refers to the use of prescription opioids that were initially intended to treat an acute pain condition and continued across the recovery phase [x], typically for more than 90 days.
The perioperative model offers an ideal context to study the transition from acute to chronic hurting [11] (pain that lasts for longer than expected, typically more than than three–6 months [12]) and astute to long-term opioid therapy [13, xiv]. When the onset of hurting and the date of initiation of opioid therapy are known, baseline measurements tin exist collected perioperatively to examine predisposing factors. The initial time-express utilization becomes prolonged for many patients; 27% of CNCP patients on opioid therapy (months to years post-obit their first prescription) were initially prescribed opioids following surgery [15]. Recent guidelines on the management of postoperative pain [16] recognize the demand for the round-the-clock analgesia over the first 24 h postoperatively and oft longer for major surgeries. The guidelines advocate for specific actions to facilitate hospital belch of patients on opioid therapy but recognize that in that location is insufficient noesis of how to perform a postoperative opioid taper [sixteen]. This lack of noesis nearly likely contributes to the unplanned evolution of prolonged opioid therapy. Considering that 2.5 million surgeries are performed yearly in Canada [17], thousands of these patients are potentially at risk for transition to prolonged and long-term opioid therapy.
Similar to the perioperative model, trauma is often associated with high intensity acute hurting, and more than than one-half of trauma patients are discharged from the hospital with an opioid prescription [10, 18]. Incidence of long-term opioid therapy following trauma may be high [nineteen,20,21,22]. For instance, 39% of workers who filed a bounty claim and who received an opioid prescription remained on opioid therapy ane year later [23]. Amongst patients with fractures, more than 40% and xxx% continue taking prescribed opioids 6 and 12 months post-fracture, respectively [19, 24].
Understanding how an initially time-limited opioid prescription turns into a long-term therapy is essential to optimize treatment and minimize potential damage associated with opioid therapy. To date, we accept found simply one narrative review on opioid consumption after surgery [25] that briefly discusses the incidence and take chances factors for long-term opioid therapy after surgery and emphasizes several strategies to facilitate opioid cessation. However, the authors omitted several relevant studies from this review [26,27,28]. There was no disquisitional appraisement of the literature or attempt to integrate results beyond the studies. This work does not examine the long-term opioid therapy in other contexts such as trauma. Lastly, considering the scientific search method is not reported, the review is subject to significant influence from the writers' opinions [29].
There is a famine of literature supporting effectiveness of long-term opioid therapy [30] and its potential negative consequences [31, 32]. As such, strategies promoting opioid-related harm reduction must target gamble factors for transitioning to long-term opioid therapy. This can only exist based on a strong understanding of how opioid therapy becomes prolonged in the commencement identify. A systematic review of the relative frequency of and the risk factors for long-term opioid therapy among populations for which opioids are oft used (e.g., fourscore% of surgical patients [3]) would generate such agreement. Due to the heterogeneity in the literature, information technology has been difficult to come to a specific conclusion. There is no consensus on what constitutes the most common risk and protective factors for the long-term utilise of opioids. The incidence of prolonged opioid use in different populations remains unknown [25]. Knowledge of what constitutes modifiable risk factors for unnecessary long-term opioid therapy is of vital importance to the prevention of secondary opioid damage. This systematic review seeks to contribute to the cognition of harm prevention; therefore, we present here details of the systematic review protocol.
Goals of systematic review
The ii master objectives of this systematic review are to:
- (1)
Examine the relative frequency of prolonged and chronic opioid therapy
and
- (ii)
Identify the adventure factors for transitioning to prolonged and chronic opioid therapy among developed patients who have experienced physical trauma requiring hospitalization and/or undergone surgery of any blazon within a hospital setting and who received an opioid prescription at discharge or within the 2 weeks post-obit infirmary belch.
Data will be examined separately for opioid-naive patients and patients on opioid therapy prior to surgery/trauma. If data permit, the relative frequency and risk factors volition exist examined in distinct populations (e.m., women, elderly, young adults, ethnic people, and individuals suffering from mental illness).
Methods
This protocol and its proposed methodology are based on Preferred ReportIng Systematic Reviews and Meta-Assay Protocols (PRISMA-P) statement [33] on the conduct of systematic review and meta-assay, the MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies [34], and the Cochrane Handbook for Systematic Review of Interventions [35]. The systematic review is composed of seven steps (see Fig. 1).
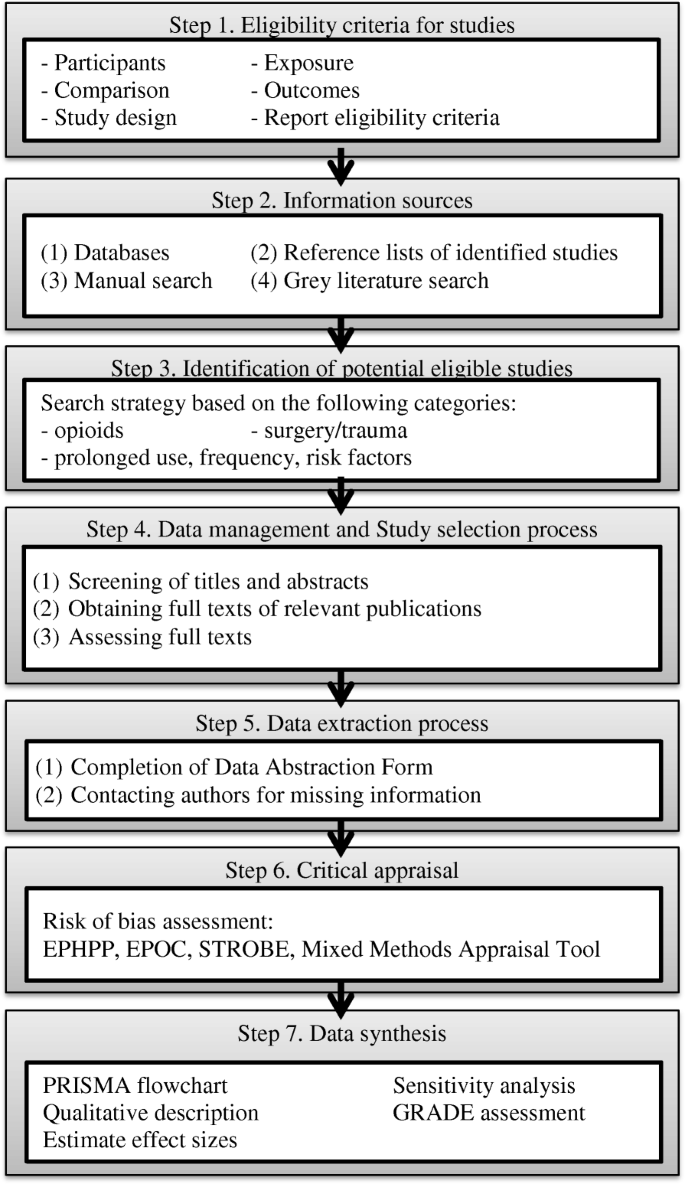
Steps of systematic review
Operationalization of key constructs
-
Surgery refers to any minor or major operative or transmission procedure involving instrumental manipulation of the living human being tissues (performed past a surgeon [36] or a not-surgical specialty). Only surgeries typically performed in-infirmary (day surgery or inpatient surgery) will be included.
-
Trauma refers to an injury to the living tissue (eastward.chiliad., body wound) that is caused by an external agent (blunt force or penetrating) [37] or other physical stressors (east.g., falls, burns, and fractures) which may or may not require any further surgical intervention. Purely psychological traumas without a physical component will not exist considered for inclusion.
-
Opioids refer to a class of drugs targeting opioid receptors. Consistent with other systematic reviews, all opioid agonists/partial agonists will exist considered, administered using pure or mixed formulations, and all systemic routes of administration will be included (routes of assistants can change over fourth dimension).
-
Opioid therapy elapsing and frequency: categorization of opioid consumption will be based on a temporal definition (every bit opposed to dose-related). Nosotros will report the frequency of use as a number of prescriptions per considered period.
-
○ Transient volition be divers as at to the lowest degree ane opioid prescription (prescribed, distributed, or reported) of whatever length within the first 44 days post discharge and at least 1 prescription of opioids between ane.5 and < 3 months (45 to 89 days) post infirmary discharge from the surgery/trauma.
-
○ Prolonged opioid therapy will be defined as at least i prescription (prescribed, distributed, or reported) inside the commencement 44 days post discharge, at least one prescription of opioids between 1.five and iii months post hospital discharge and at to the lowest degree one prescription of opioids of any length betwixt 3 and 6 months post hospital discharge from the surgery/trauma, irrespective of the dose.
-
○ Chronic opioid therapy volition be defined equally at least one opioid prescription (prescribed, distributed or reported) of whatsoever length more than than half-dozen months post hospital belch post-obit surgery/trauma in addition to coming together criteria for prolonged opioid therapy.
-
These definitions are based on time-to-cessation [38] of opioids following surgery. Long-term opioid therapy is used in this article to comprehend both prolonged and chronic opioid apply (a generic term describing opioid consumption that exceeds xc days following infirmary belch post-event).
-
Daily dosing: in that location is no consensus on definitions of lower versus high-dose therapy [39]. Fifty-fifty morphine equivalence daily doses (MEDD) between 20 and fifty mg are associated with increased risk of overdose and death [40]. When information are available, we volition convert reported daily doses for long-term opioid therapy into their respective MEDD. Daily opioid consumption volition be reported equally follows:
-
○ Low dose defined equally a total daily dose less than 50 MEDD
-
○ Moderate dose defined every bit a total daily dose betwixt 50 and 90 MEDD
-
○ High dose defined every bit a total daily dose above ninety MEDD
-
-
Type of opioid use will be defined as episodic or continuous
-
○ Episodic use is opioid consumption > 3 months with total days of opioid supply < 120, and total number of prescriptions filled < 10
-
○ Continuous apply is opioid consumption > 3 months with ≥120 days of opioid supply and ≥ 10 total number of prescriptions filled [5, 41, 42].
-
-
Preoperative and pre-trauma opioid use condition: patients volition be classified equally preoperative or pre-trauma opioid users if they have received opioid supplies more than one month before surgery or trauma. Patients who received opioids only in the days before surgery (30 days or less) will be considered equally opioid naive given that opioids are ofttimes prescribed to patients before surgery for postoperative pain control.
Eligibility criteria
Eligibility criteria are defined based on the PICOS (Participants, Intervention/Exposition, Control, Outcomes, and Report pattern) approach [43]. Study pick will be based on the criteria outlined below.
Participants
Inclusion criteria
Studies will exist included if they meet the following criteria: adults (≥ 18 years old), surgery (minor/major performed at the hospital) and/or physical trauma/injury (requiring hospitalization) and requiring opioid-based analgesia of whatever duration post-infirmary belch (at least one opioid prescription filled at discharge or within ii weeks of infirmary discharge). Consistent with other reviews, in case of mixed populations (e.one thousand., a study that has included some cancer patients within their sample) for which information cannot exist obtained by authors, studies with ≥ 75% of patients coming together the inclusion criteria will be accepted [41]. Purely psychological traumas without a physical component will non be considered for inclusion. Methadone and buprenorphine are almost never starting time-line analgesic agents; however, they will be included if time for initiation and analgesic purpose are clearly identified in a report. Since the principles of pediatric analgesia rely on differing therapeutic criteria [44, 45], only adults are included in this review.
Exclusion criteria
Studies will exist excluded if whatsoever of the post-obit conditions apply: study participants suffering from cancer pain, requiring end-of-life intendance, with addiction substitution/maintenance therapy, with lack of consideration for presence/absence of opioid therapy pre-surgery/trauma, and having undergone another surgical process or experienced another trauma during the study follow-upwards period. Studies in which a fourth dimension frame for opioid initiation and handling duration cannot be identified volition be excluded. In cases where studies examine only the 3-month outcomes with no mention of opioid intake betwixt discharge and follow-upward, these studies will be included if information is available to decide that the opioid prescription at 3 months is taken because of the indexed result. Given that opioid-naive patients and patients on opioid therapy earlier surgery or trauma will exist examined separately, studies with mixed populations that do non allow for subgroup analyses and the examination of incidence and risk factors separately (eastward.g., for opioid-naive patients and preoperative/pre-trauma opioid users) will be excluded.
Exposure
Exposure will consist of having received at least 1 opioid prescription inside 2 weeks post-obit hospital belch post-event.
Comparison
Comparing, when applicable, would consist of comparison the presence/absenteeism or degree of risk factors between patients who developed long-term opioid therapy vs. those who discontinued opioids in the astute phase.
Outcomes
Objective 1
Frequency of opioid use, daily dosing, and type of opioid utilise at dissimilar fourth dimension points (45 days, 90 days, 6 months, and 12 months post-obit hospital discharge postal service event) volition be reported for each blazon of surgery and trauma. At that place must be an show of electric current opioid use (i.e., opioid prescription filled within that time window) at the pre-determined cut-off points for transient, prolonged, and chronic opioid therapy and no menses greater than 90 days without opioid consumption at any signal since hospital belch [46]. This data will have been sourced from the medico-administrative databases or medical charts, or patient self-report information.
Objective two
In the identification of biopsychosocial take chances and protective factors for long-term opioid therapy mail-discharge, three types of risk factors will be assessed related to (1) patients, (2) health care providers, and (iii) wellness care system. Data sources for patient factors will include validated self-report questionnaires as well as medical charts/medico-authoritative databases. Data sources for wellness care providers and wellness intendance organisation factors will include medical charts, md-authoritative databases, and clinical/health care systems.
Study design
Inclusion
The post-obit study designs volition exist included: experimental designs (randomized controlled trial, quasi-experimental designs), observational studies (cross-sectional, accomplice, example-control, example series), and mixed methods studies.
Exclusion
We will exclude editorials and commentaries. Studies with a follow-up period shorter than 1.5 months following hospital discharge post-event will be excluded.
Limits
Language: Merely manufactures published in English or French will be considered.
Publication status and publication year: Articles published since 1998 or in printing will be considered for inclusion. This cut-off was chosen given that it follows the first consensus argument by the American Academy of Pain Medicine and the American Pain Club on the use of opioids in the treatment of chronic non-cancer pain [47] leading to significant do change in the employ of opioids for CNCP management.
Information sources
The following four literature categories volition be searched:
- ane.
Databases: The post-obit databases will exist searched by a professional person report librarian (DZ) and reviewed past 2 independent librarians: MEDLINE (1998–), PubMed (1998–), CINAHL (1998–), PsycINFO (1998–), EMB Review (1998–), EMBASE (1998-), Cochrane Database of Systematic Reviews, and Cochrane Cardinal Register of Controlled Trials.
- 2.
Reference lists of identified studies and whatsoever relevant review manufactures institute will exist screened;
- three.
Additional manual search of relevant journals peculiarly for trauma given the variety of key words used for indexing (Trauma and Acute Intendance, Register of Emergency Medicine, Emergency Medicine International, European Journal of Trauma & Emergency Surgery);
- 4.
Grey literature (Google scholar, Pro Quest Dissertation and Theses; published reports).
The search bibliography will also be circulated to all study authors who accept expertise in the domain.
A focused search for distinct populations (elderly, women, young adults, indigenous people, and individuals suffering from mental illness) volition also be performed using Google Scholar. To conduct this specific search, we will add key terms (e.1000., Ethnic) to the search strategy in Google Scholar and compare the manufactures retrieved to those found in the original search. We will continue until 10 pages with the maximum number of articles take been reviewed or until saturation signal has been reached (when Google Scholar does not identify any articles not previously included), whichever happens first.
Search strategy
The search strategy was elaborated past our information expert (librarian) (DZ) and our field experts (GP, IK) and reviewed by a 2d independent librarian. We have reviewed central words, MeSH terms, and search strategies used in relevant original studies and literature reviews. We program to behave the search for the relevant studies based on the following terms and derived terminology from the iv pre-identified conceptual groups: (1) opioid (including synonyms, generic, and brand names of medications); (ii) surgery or trauma (including names of specific weather typically not indexed nether these terms, e.g., knee arthroplasty, fracture, and burn down); (three) transient/prolonged/long-term/frequency/prevalence/incidence or hazard factors. The complete search strategy for MEDLINE database is presented in the Appendix. These search terms volition be adapted for each of the databases to be considered. For each of the databases, controlled vocabulary (MeSH, EMTREE, and others), and gratuitous-text searching will be used. A transmission search of the bibliographies of each of the original studies and relevant reviews included volition be conducted by GP and research assistants to identify other potential relevant references.
Study records
Data management
All search results will exist merged using the EndNote software, and indistinguishable records will exist removed. The written report selection process volition be performed using the software DistillerSR (DistillerSR, Bear witness Partners, Ottawa, Canada). Titles and abstracts will be independently screened for eligibility past ii reviewers. Reviewers will include GP, IK, and experienced research assistants. The full text of potentially relevant reports volition exist further analyzed for eligibility. Disagreements volition exist resolved by consensus and if needed, past requesting the opinion of the tertiary reviewer. To ensure consistency across reviewers, a review of selection procedure volition exist performed after ten% of the identified articles take been reviewed. Levels of inter-rater agreement [48] (kappa statistics) will be documented.
Selection process
A research assistant will obtain the full text of all relevant publications and further analyze the data against the defined eligibility criteria. Two reviewers will exist assigned to each publication, and input from the tertiary reviewer volition exist solicited in case of disagreement. The following information volition be recorded in this modified Effective Practice and Organisation of Care (EPOC) course: surname of first author, year of beginning report, engagement course completed, names of reviewers extracting data, report title, type of publication, funding source, conflicts of interests, and report characteristics (study blazon, participant, intervention types, and outcomes).
A manual search using authors' names volition be conducted to place unmarried studies that accept been published more than once. The review authors volition not be blinded to journal titles and study authors of institutions.
Data extraction and reporting
For the data extraction of selected studies, we volition adapt the Cochrane EPOC Data Brainchild Form and Information Extraction Instructions [49]). Data extraction will exist washed inside the DistillerSR software. For the specific needs of this systematic review, GP and the research assistant will pilot the adapted EPOC course with instructions on 10 randomly chosen studies to refine and finalize information technology.
Information extraction will be independently done by GP and research administration using the adjusted recording form. The data extraction form sections are designed to excerpt data concerning all aspects of each study, including population and study characteristics, methods used to mensurate exposition and issue characteristics, results on association measures of involvement, and results applicability. Authors will exist contacted as needed to obtain missing information.
Patient data will be organized into the broad categories of risk factors like to those for chronic postoperative pain [11], namely sociodemographic, psychosocial, and medical/surgical/trauma characteristics. Health care professional person take chances factors will be categorized into sociodemographic, psychosocial, and professional (e.thousand., experience, type of profession, and prescribing practices). Lastly, health intendance system take a chance factors will be categorized into infrastructure (e.k., proximity of pharmacies and access to health care professional) and economic (e.g., percent of reimbursement of opioid prescription costs).
Data items and outcomes
Objective 1: relative frequency of opioid therapy
Primary outcome
The primary outcome will be the relative frequency of long-term opioid therapy (≥ xc days) post-obit hospital discharge post trauma and/or mail service surgery. This will be reported as a proportion of patients on opioid therapy out of the full number of eligible patients (refer to the section "Operationalization of fundamental constructs" for more details).
Secondary outcomes
Secondary outcomes will examine the relative frequency of opioid therapy at different stages post-obit infirmary discharge (transient, prolonged, and chronic utilize periods). Relative frequencies will exist examined based on preoperative/pre-trauma opioid status and if data permits based on small vs. major surgeries [50].
Objective 2: risk and protective factors for long-term opioid therapy
3 types of run a risk factors volition exist assessed (patients, wellness care providers, and health care system).
Chief issue
Identification of biopsychosocial risk and protective factors for long-term opioid therapy mail service-discharge (≥ 90 days).
Secondary outcomes
In identification of biopsychosocial risk and protective factors at the different stages post-obit hospital belch, some literature suggests that the risk factors for the development (3-month status) vs. maintenance (12-month status) of postoperative pain are not the same (due east.g., [51]). As such, as data permits, this review will examine risk factors for prolonged opioid use separately from chronic opioid use, the latter allowing for run a risk factors measured 3 months later infirmary belch to exist examined.
Risk of bias in private studies
Two from the v reviewers (GP, IK, research administration) will review each included commodity separately to assess the risk of bias for each outcome of interest. In example of disagreement, a word will have place to achieve consensus; otherwise, a third reviewer volition appraise the study. Dissimilar assessment tools will exist used depending on each report design: the Effective Public Wellness Practice Project (EPHPP) Quality Assessment Tool for Quantitative Studies for cohort studies and case-control studies [52], the EPOC Hazard of Bias Tool for RCT [53], the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [54], and Mixed Methods Appraisal Tool for Systematic Mixed Studies Reviews [55]. 8 categories (option, written report design, confounders, blinding, data drove methods, withdrawals/drop-outs, integrity of delivered intervention, analyses) will be assessed using 21 items. The EPHPP tool has a good inter- and intra-rater reliability [52, 56] and is one of the top tools for disquisitional appraisal in systematic reviews [57]. Source of report funding (eastward.g., sponsored by pharmaceutical companies or not) will also be examined.
Synthesis
Data synthesis process
First, results of the study selection process volition exist described using a PRISMA Flowchart [58], and the statistic of inter-rater agreement (kappa) [48] will be reported. Second, we will perform a qualitative description of the population, the studies included, the risk factors identified, and the upshot characteristics using unproblematic frequency counts and a narrative approach. Third, only the studies in which data on risk factors and the outcomes of interest are bachelor to estimate the relative frequency and effect size volition be included in the meta-analysis.
Data synthesis reporting
If effect sizes are available or calculable in two or more studies for a specific outcome, a meta-assay will be conducted using the software Review Director [59]. For continuous outcomes, nosotros will employ standardized mean difference (SMD) with 95% CI, and for dichotomous outcomes, we will use the relative risk (RR) with 95% CI. Adding and/or transformation of consequence sizes into RR or SMD will be done when possible. When issue sizes cannot exist calculated or when merely one effect size is available for an event, we volition report the results of this issue every bit a narrative synthesis. Due to the predictable heterogeneity of the information, we will use a random-effects model to puddle the effect size of the adventure factor for each upshot [60]. Only the adjusted issue sizes will be considered in this model. Nosotros will besides calculate Higgins' I 2 statistic, i.e., the per centum of variability in the outcome size estimates due to the heterogeneity [61]. The chi-squared test will be used to test the heterogeneity [61, 62]. Moreover, the potential heterogeneity (i.e., I 2 ≥ 50%) volition be explored using subgroup analyses based on studies, participants, and exposure characteristics mentioned above.
If sufficient information are available to examine RR and gamble factors for long-term opioid therapy amidst individuals with low socioeconomic status, women, youth, ethnic people, and individuals with a diagnosed mental disease, we volition summarize and synthesize the data in a narrative fashion.
Meta-biases
For clinical trials published after 2005, reporting bias will be assessed past determining whether a protocol for the selected articles was published prior to data collection. We will besides assess the publication bias for each outcome by visually examining funnel plots when more than than ten studies are included in the meta-assay [35]. Finally, we plan to perform the following sensitivity analyses in guild to assess the robustness of our results on each consequence of involvement: (i) nosotros volition explore the individual influence of each study by sequentially removing i at the time from the pooled effect size interpretation, (2) we will repeat the pooled effect size estimation by including only studies with depression risk of bias, and (3) as we anticipate that misreckoning variables may vary depending on which studies are included, we will approximate the pooled crude effect size for each outcome using crude effect sizes (non-adjusted) merely. Results of these analyses will be compared with the initial pooled effect sizes in society to appraise the impact of the confounding variables.
Confidence in cumulative evidence
For each effect, we volition assess the quality of cumulative evidence with the Grading of Recommendations Assessment, Development and Evaluation (Grade) [63] to reduce the misinterpretation of the results of the review. This tool is based on v criteria such every bit the adventure of bias in each individual study, the indirectness of the prove, the heterogeneity of the data, the imprecision of the effect size estimates, and the risk of publication bias. The quality of evidence will be rated high, moderate, low, or very low. Equally mentioned previously, we volition as well examine the take a chance of bias associated with a funding source.
Discussion
The outcomes of this systematic review will directly inform health care policies and could potentially change the clinical practise of multiple wellness professionals who are involved in the initial prescriptions of opioids (e.g., anesthesiologists, surgeons, emergency physicians, obstetricians, and physiatrists). The results of this review will modify approach to prolonged and chronic opioid therapy among the providers involved in the renewal and discontinuation of opioid therapy (e.thou., pharmacists, primary care physicians, orthopedic surgeons, and psychiatrists), and those practicing at the primary, perioperative and trauma (post-acute treatment decision) care levels. The agreement of the underlying risks for long-term opioid therapy will ultimately contribute to the cost-effect optimization of the patient monitoring processes in the days/weeks following the initiation of opioid therapy. The synthesis data will inform clinical algorithms and help to develop appropriate risk prevention programs.
References
-
Fischer B, Keates A, Buhringer G, Reimer J, Rehm J. Non-medical use of prescription opioids and prescription opioid-related harms: why and then markedly higher in N America compared to the balance of the world. Addiction. 2014;109(two):177–81.
-
Berterame S, Erthal J, Thomas J, Fellner S, Vosse B, Clare P, Hao W, Johnson DT, Mohar A, Pavadia J, et al. Apply of and barriers to admission to opioid analgesics: a worldwide, regional, and national study. Lancet. 2016;387(10028):1644–56.
-
Wunsch H, Wijeysundera DN, Passarella MA, Neuman Physician. Opioids prescribed after depression-risk surgical procedures in the United states of america, 2004–2012. JAMA. 2016;315(15):1654–seven.
-
Haanpaa ML, Backonja MM, Bennett MI, Bouhassira D, Cruccu One thousand, Hansson PT, Jensen TS, Kauppila T, Rice Every bit, Smith BH, et al. Cess of neuropathic pain in primary care. Am J Med. 2009;122(10 Suppl):S13–21.
-
Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan Md, Rutter CM, Silverberg MJ, Banta-Dark-green C, et al. De facto long-term opioid therapy for noncancer pain. Clin J Hurting. 2008;24(6):521–7.
-
Bates C, Laciak R, Southwick A, Bishoff J. Overprescription of postoperative narcotics: a await at postoperative pain medication delivery, consumption and disposal in urological exercise. J Urol. 2011;185(2):551–v.
-
Brown MT, Bussell JK. Medication adherence: WHO cares. Mayo Clin Proc. 2011;86(four):304–14.
-
Rodgers J, Cunningham Grand, Fitzgerald Thousand, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Manus Surg Am. 2012;37(4):645–l.
-
Canadian Institute for Health Information. How Canada compares: results from the Commonwealth Fund's 2016 International Wellness Policy Survey of Adults in 11 Countries—accessible report. Ottawa: CIHI; 2017.
-
Clarke H, Soneji Northward, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid utilise after major surgery: population based cohort written report. Bmj. 2014;348:g1251.
-
Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: chance factors and protective factors. Adept Rev Neurother. 2009;9(5):723–44.
-
Werner MU, Kongsgaard UE. I. defining persistent post-surgical pain: is an update required. Br J Anaesth. 2014;113(i):1–4.
-
Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain post-obit surgery by developing a transitional pain service. Pain Manag. 2015;5(ii):97–105.
-
Huang A, Azam A, Segal S, Pivovarov K, Katznelson G, Ladak SS, Mu A, Weinrib A, Katz J, Clarke H. Chronic postsurgical pain and persistent opioid use following surgery: the need for a transitional pain service. Pain Manag. 2016;6(5):435–43.
-
Callinan CE, Neuman Dr., Lacy KE, Gabison C, Ashburn MA. The initiation of chronic opioids: a survey of chronic pain patients. J Pain. 2017;18(4):360–five.
-
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, et al. Management of postoperative pain: a clinical practice guideline from the American Hurting Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Commission, and Administrative Council. J Pain. 2016;17(two):131–57.
-
Canadian Institute for Health Information. Surgical volume trends, 2009: Within and beyond wait time priority areas. 2009. Ottawa, ON, Canada: CIHI: 44p. [https://secure.cihi.ca/free_products/surgical_volumes_2009_e.pdf].
-
Chaudhary MA, Schoenfeld AJ, Harlow AF, Ranjit A, Scully R, Chowdhury R, Sharma Grand, Nitzschke Due south, Koehlmoos T, Haider AH. Incidence and predictors of opioid prescription at discharge later on traumatic injury. JAMA Surg. 2017;152(10):930–half dozen.
-
Al Dabbagh Z, Jansson KA, Stiller CO, Montgomery S, Weiss RJ. Long-term pattern of opioid prescriptions later on femoral shaft fractures. Acta Anaesthesiol Scand. 2016;lx(5):634–41.
-
Johnson SP, Chung KC, Zhong L, Shauver MJ, Engelsbe MJ, Brummett C, Waljee JF. Risk of prolonged opioid utilise among opioid-naive patients following common mitt surgery procedures. J Hand Surg Am. 2016;41(ten):947–57. e943
-
Zwisler ST, Hallas J, Larsen MS, Handberg G, Mikkelsen South, Enggaard TP. Opioid prescriptions before and after high-energy trauma. J Opioid Manag. 2015;xi(4):313–8.
-
Alghnam S, Castillo R. Traumatic injuries and persistent opioid use in the The states: findings from a nationally representative survey. Inj Prev. 2017;23(two):87–92.
-
Berecki-Gisolf J, Collie A, McClure RJ. Prescription opioids for occupational injury: results from workers' compensation claims records. Hurting Med. 2014;fifteen(ix):1549–57.
-
Weiss RJ, Montgomery SM, Stiller C-O, Wick MC, Jansson K-A. Long-term follow-up of opioid apply in patients with acetabular fractures. Injury Extra. 2012;43:49–53.
-
Hah JM, Bateman BT, Ratliff J, Curtin C, Sun Eastward. Chronic opioid use after surgery: implications for perioperative management in the face up of the opioid epidemic. Anesth Analg. 2017;125(v):1733–40.
-
Lawrence JTR, London N, Bohlman HH, Chin KR. Preoperative narcotic use equally a predictor of clinical consequence. Spine. 2008;33(19):2074-viii.
-
Rozet I, Nishio I, Robbertze R, Rotter D, Chansky H, Hernandez AV. Prolonged opioid utilize after knee arthroscopy in armed services veterans. Anesth Analg. 2014;119(2):454–nine.
-
Schoenfeld AJ, Nwosu K, Jiang W, Yau AL, Chaudhary MA, Scully RE, Koehlmoos T, Kang JD, Haider AH. Risk factors for prolonged opioid use following spine surgery, and the association with surgical intensity, among opioid-naive patients. J Bone Joint Surg Am. 2017;99(15):1247–52.
-
Mulrow CD. Rationale for systematic reviews. BMJ. 1994;309(6954):597–nine.
-
Chou R, Deyo R, Devine B, Hansen R, Sullivan S, Jarvik JG, Blazina I, Data T, Bougatsos C, Turner J. The effectiveness and risks of long-term opioid treatment of chronic pain evidence report/technology cess, vol. 218. Agency for Healthcare Inquiry and Quality: Rockville; 2014.
-
Baldini A, Von Korff M, Lin EH. A review of potential agin effects of long-term opioid therapy: a practitioner's guide. Prim Care Companion CNS Disord. 2012;xiv(3)
-
Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic hurting: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(four):276–86.
-
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Grouping P-P. Preferred reporting items for systematic review and meta-assay protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
-
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
-
Higgins JPT, Dark-green South. Cochrane handbook for systematic reviews of interventions Version 5.one.0. In: Higgins J, Green South, editors. The Cochrane Collaboration, vol. 2014; 2011.
-
"Surgery." Merriam-Webster.com. Merriam-Webster, n.d. Web. 4 July 2018. [https://www.merriam-webster.com/lexicon/surgery]
-
"trauma." Merriam-Webster.com. Merriam-Webster, n.d. Web. four July 2018. [https://www.merriam-webster.com/dictionary/surgery]
-
Mudumbai SC, Oliva EM, Lewis ET, Trafton J, Posner D, Mariano ER, Stafford RS, Wagner T, Clark JD. Time-to-cessation of postoperative opioids: a population-level analysis of the veterans affairs health care arrangement. Pain Med. 2016;17(nine):1732–43.
-
Kobus AM, Smith DH, Morasco BJ, Johnson ES, Yang 10, Petrik AF, Deyo RA. Correlates of higher-dose opioid medication use for low dorsum pain in principal intendance. J Pain. 2012;13(11):1131–eight.
-
Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—The states, 2016. MMWR Recomm Rep. 2016;65(ane):1–49.
-
Chou R, Huffman L. Use of chronic opioid therapy in chronic noncancer pain: show review. In: The American Pain Society in Conjunction with The American Academy of Pain Medicine; 2009.
-
Busse JW, Craigie S, Juurlink DN, Buckley N, Wang L, Couban RJ, Agoritsas T, Akl EA, Carrasco-Labra A, Cooper LK, et al. Guideline for opioid therapy and chronic noncancer hurting. Can Med Assoc J. 2017;189:E659–66.
-
O'Connor D, Dark-green Southward, Higgins JPT. Chapter 5: Defining the review question and developing criteria for including studies. In: Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. edn. Edited past Higgins J, Greenish S: The Cochrane Collaboration. England: Wiley; 2011.
-
Berde CB, Sethna NF. Analgesics for the handling of pain in children. N Engl J Med. 2002;347(14):1094–103.
-
Howard RF. Current condition of pain management in children. JAMA. 2003;290(18):2464–9.
-
Soneji Due north, Clarke HA, Ko DT, Wijeysundera DN. Risks of developing persistent opioid use after major surgery. JAMA Surg. 2016;151(11):1083–iv.
-
Haddox JD, Joranson DE, Angarola RT, Brady A, Carr DB, Blonsky ER, Burchiel K, Gitlin Chiliad, Midcap M, Payne R, et al. The use of opioids for the treatment of chronic pain: a consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain. 1997;13(one):6–viii.
-
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Montclair NJ: Lawrence Erlbaum Associates; 1988.
-
Cochrane Effective Practice and Organisation of Intendance (EPOC). EPOC resource for review authors. 2017 Bachelor at: [http://epoc.cochrane.org/epoc-specific-resource-review-authors].
-
Minor RG, Witt RE. Major and modest surgery. JAMA. 1965;191:180–2.
-
Page MG, Stinson J, Campbell F, Isaac 50, Katz J. Identification of pain-related psychological risk factors for the evolution and maintenance of pediatric chronic postsurgical pain. J Pain Res. 2013;6:167–80.
-
Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research testify for public health nursing interventions. Worldviews Evid-Based Nurs. 2004;1(iii):176–84.
-
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman Advertisement, Savovic J, Schulz KF, Weeks Fifty, Sterne JA, et al. The Cochrane Collaboration's tool for assessing hazard of bias in randomised trials. Bmj. 2011;343:d5928.
-
Quality Assessment Tool for Observational Cohort and Cantankerous-Sectional Studies [http://mixedmethodsappraisaltoolpublic.pbworks.com/w/page/24607821/FrontPage].
-
Pluye P, Robert Eastward, Cargo M, Bartlett G, O'Cathain A, Griffiths F, Boardman F, Gagnon MP, Rousseau MC: Proposal: a mixed methods appraisal tool for systematic mixed studies reviews. 2011.
-
Armijo-Olivo South, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Cess of study quality for systematic reviews: a comparison of the Cochrane collaboration take chances of bias tool and the constructive public health exercise projection quality assessment tool: methodological inquiry. J Eval Clin Pract. 2012;18(ane):12–8.
-
Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. International Stroke Trial Collaborative Grand, European Carotid Surgery Trial Collaborative Grand: evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii–x. ane–173
-
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate wellness care interventions: caption and elaboration. PLoS Med. 2009;6(seven):e1000100.
-
Cochrane Collaboration: Review Managing director (RevMan) Version 5.2. 2012.
-
Borestein Yard, Hedges LV, Higgins JPT, Rothstein 60 minutes. Introduction to meta-analysis. Chichester: Wiley-Blackwell; 2009.
-
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
-
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–threescore.
-
Guyatt GH, Oxman Advertizing, Schunemann HJ, Tugwell P, Knottnerus A. Course guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2.
Acknowledgements
Caroline Sauvé, librarian at the Department of Information science of the Hôtel Dieu, Center hospitalier de fifty'Université de Montréal has contributed to the elaboration of the search strategy in MEDLINE.
Funding
This systematic review is funded and sponsored by the Canadian Institutes of Health Research Operating Grant: Opioid Crunch Knowledge Synthesis # 397974. Through funding, peer review process reviewers have provided comments that were incorporated into this manuscript. The written report team has received in-kind contribution from the "Health and Social Services Systems, Knowledge Translation and Implementation" component of the Quebec SPOR-Support Unit.
Amendments
If amendments are needed to this protocol as we motion forward with the review, we will provide the date of each amendment as well as a description and rationale of each change fabricated when publishing the review.
Writer information
Affiliations
Contributions
All study authors contributed intellectually to the evolution of the nowadays protocol. GP and IK wrote the manuscript. All authors reviewed and approved the final version of this manuscript. GP is the report guarantor.
Corresponding author
Ideals declarations
Ideals approving and consent to participate
Ethics approval is not required for systematic reviews.
Consent for publication
Not applicable.
Competing interests
The authors declare that they accept no competing interests.
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Search Strategy
Database: Ovid MEDLINE® in-process and other not-indexed citations, Ovid MEDLINE®, 1946 to present.
| 1 | Thoracic Surgery/ |
| 2 | Surgery Department, Hospital/ |
| three | Gynecologic Surgical Procedures/ |
| 4 | Convalescent Surgical Procedures/ |
| v | Surgical Procedures, Operative/ |
| half-dozen | Cardiovascular Surgical Procedures/ |
| 7 | Digestive Organization Surgical Procedures/ |
| 8 | Elective Surgical Procedures/ |
| 9 | Endocrine Surgical Procedures/ |
| 10 | Minor Surgical Procedures/ |
| eleven | Neurosurgical Procedures/ |
| 12 | Obstetric Surgical Procedures/ |
| 13 | Ophthalmologic Surgical Procedures/ |
| 14 | Orthopedic Procedures/ |
| 15 | Otorhinolaryngologic Surgical Procedures/ |
| xvi | Perioperative Intendance/ |
| 17 | Reconstructive Surgical Procedures/ |
| 18 | Thoracic Surgical Procedures/ |
| 19 | Cardiovascular Arrangement/su [Surgery] |
| 20 | Bariatric Surgery/ |
| 21 | "Wounds and Injuries"/ |
| 22 | Intestinal Injuries/ |
| 23 | Amputation, Traumatic/ |
| 24 | Arm Injuries/ |
| 25 | Back Injuries/ |
| 26 | BURNS/ |
| 27 | Common cold Injury/ |
| 28 | Contrecoup Injury/ |
| 29 | Trounce Injuries/ |
| 30 | Electrical Injuries/ |
| 31 | Fractures, Bone/ |
| 32 | Hand Injuries/ |
| 33 | Hip Injuries/ |
| 34 | Joint Dislocations/ |
| 35 | Leg Injuries/ |
| 36 | Neck Injuries/ |
| 37 | Occupational Injuries/ |
| 38 | Shoulder Injuries/ |
| 39 | Soft Tissue Injuries/ |
| xl | "Sprains and Strains"/ |
| 41 | Tendon Injuries/ |
| 42 | Thoracic Injuries/ |
| 43 | Peripheral Nerve Injuries/ |
| 44 | Vascular System Injuries/ |
| 45 | War-Related Injuries/ |
| 46 | Wounds, Nonpenetrating/ |
| 47 | Wounds, Penetrating/ |
| 48 | POSTOPERATIVE CARE/ or POSTOPERATIVE Catamenia/ or Pain, Postoperative/ |
| 49 | (injur* or surger* or surgical or traum* or arthroplast* or accident* or fracture* or dislocation*).tw,sh,kw,kf,oa. |
| l | or/1-49 |
| 51 | exp Analgesics, Opioid/ |
| 52 | exp CODEINE/ |
| 53 | exp MORPHINE/ |
| 54 | CODEINE/ |
| 55 | FENTANYL/ |
| 56 | HYDROCODONE/ |
| 57 | HYDROMORPHONE/ |
| 58 | MEPERIDINE/ |
| 59 | OXYCODONE/ |
| 60 | exp NARCOTICS/ |
| 61 | BUPRENORPHINE/ |
| 62 | BUPRENORPHINE, NALOXONE DRUG COMBINATION/ |
| 63 | METHADONE/ |
| 64 | OXYCODONE/ |
| 65 | OXYMORPHONE/ |
| 66 | PENTAZOCINE/ |
| 67 | TRAMADOL/ |
| 68 | LEVORPHANOL/ |
| 69 | (actiq or adolonta or amadol or analgesic* or anpec or ardinex or asimadolin* or astramorph or avinza or biodalgic or bpethidine or buprenorphine or carfentanil or codeine or codinovo or contramal or demerol or dicodid or dihydrocodeinone or dihydrohydroxycodeinone or dihydromorphinone or dihydrone or dilaudid or dinarkon or dolantin or dolargan or dolcontral or dolosal or dolsin or dur?gesic or dur?morph or epimorph or eucodal or exalgo or fentanest or fentanyl or fentora or fortral or hycodan or hycon or hydrocodeinonebitartrate or hydrocodone or hydrocodone* or hydromorphon or hydromorphone or hydroxyacetanilide or hydroxycodeinon or hysingla or isocodeine or isonipecain or jutadol or kadian or l dromoran or laudacon or levodroman or levodromoran or levo-dromoran or levorphan or levorphanol or lexir or lidol or lorcet or lortab or lydol or meperidine hydrochloride or methadone or morfin or morfine or morphia or morphin or morphine or morphinium or morphium or ms contin or n methylmorphine or narcotic or n-methylmorphine or nobligan or norco or numorphan or operidine or opiate or opioid* or opso or oramorph sr or oripavine or oxecta or oxiconum or oxycodeinon or oxycodone or oxycone or oxycontin or oxymorphone or palladone or pancodine or pentazocine or percocet or pethidine or phentanyl or prontofort or propoxyphene or robidone or roxicet or roxicodone or skenan or sublimaze or takadol or talwin or thebaine or theocodin or theradol or tiral or topalgic or tradol or tradolpuren or tradonal or tralgiol or trama or tramadin or tramadoc or tramadol or tramadolhameln or tramadolor or tramadorsch or tramadura or tramagetic or tramagit or tramake or tramal or tramex or tramundin or trasedal or ultram or vicodin or zamudol or zohydro or zumalgic or zydol or zytram).tw,sh,kw,kf,oa,nm. |
| 70 | or/51-70 |
| 71 | ((chronic* or persistent or long-term or "long term" or prolonged or continu*) adj2 (user* or "use" or usage or pattern or consumption or therap* or event or effects)).tw,sh,kw,kf,oa. |
| 72 | PREVALENCE/ |
| 73 | INCIDENCE/ |
| 74 | Hazard Assessment/ |
| 75 | Take a chance Factors/ |
| 76 | ("long term" or prolonged or chronic*).tw,sh,kw,kf,oa. |
| 77 | ("risk factors" and ("long term" or prolonged or chronic*)).tw,sh,kw,kf,oa. |
| 78 | 76 and 77 |
| 79 | or/72-75 |
| 80 | 78 or 79 or eighty |
| 81 | 50 and 71 and 81 |
| 82 | limit 82 to twelvemonth="1998 -Current" |
| 83 | animals/ |
| 84 | humans/ |
| 85 | 84 not (84 and 85) |
| 86 | 83 not 86 |
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/past/4.0/), which permits unrestricted employ, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/nada/one.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
Nigh this article
Cite this commodity
Pagé, One thousand., Kudrina, I., Zomahoun, H. et al. Relative frequency and take a chance factors for long-term opioid therapy following surgery and trauma amid adults: a systematic review protocol. Syst Rev 7, 97 (2018). https://doi.org/ten.1186/s13643-018-0760-three
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s13643-018-0760-3
Keywords
- Systematic review protocol
- Surgery
- Trauma
- Opioids
- Take chances factors
- Prolonged therapy
- Long-term use
Source: https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-018-0760-3
Belum ada Komentar untuk "A Review of Potential Adverse Effects of Long-term Opioid Therapy"
Posting Komentar